No effective treatment exists for estimated 250,000 children born worldwide each year (20,000 in the US) with pediatric minimally verbal autism
By Dakota Orlando
New York, June 07, 2019 – Q BioMed, a commercial-stage biotechnology acceleration firm today announced advancements on several fronts for its key asset, QBM-001, in the treatment of pediatric minimally verbal autism (PMVA), a rare autism spectrum disorder for which no effective treatment currently exists. Globally, over 250,000 children are born with PMVA per year, 20,000 of them the United States.
“Children affected by pediatric minimally verbal autism are in dire need of an effective treatment,” stated Q BioMed CEO Denis Corin. “Q BioMed is committed to delivering relief to the children and families that are impacted by this condition. The emotional strain of never talking with one’s child can be severe, and the direct financial costs over a lifetime are estimated to be $4.5 million per family.”
“We are proud of the scientific breakthrough we’ve made with the discovery of RNA biomarkers correlated with PMVA. This new data has accelerated QBM-001 towards clinical development. The data improves our clinical trial design, provides valuable insight for regulators, and opens doors with new collaborators and key opinion leaders in the field,” noted Corin.
Recent achievements and upcoming milestones in QBM-001’s path to market include:
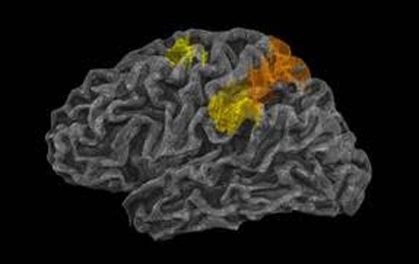
- Discovery of Biomarkers in April 2019: In April, Q BioMed announced its discovery of the first biomarkers to identify PMVA. The company’s study examined 1,953 autistic biomarkers, resulting in two significant markers tied to expressed genes and pathways responsible for speech development. These biomarkers will help identify and homogenize clinical trial participants, and will be studied as possible diagnostic and prognostic indicators during trials. Successful outcomes would allow for a diagnosis as young as 18 months.
- Attendance and enthusiastic reception at the International Society for Autism Research (INSAR) annual meeting in 2019. Q BioMed attended the largest clinical and research focused conference for autism, the International Society for Autism Research Annual Meeting, in May. The independent findings at the conference provided unique insight into PMVA and also confirmed that PMVA can be stratified as a subset from other ASDs.
- Mother with child having PMVA
- These INSAR findings, along with Q BioMed’s biomarker study, allowed the company to organize an advisory board meeting at INSAR to prepare regulatory and diagnostic guidelines for PVMA. Q BioMed is currently in discussions with clinics and research centers from the conference regarding potential partnerships and collaborations.
- Orphan Drug Application in June 2019. Q BioMed plans to apply for Orphan designation can be given to drugs that treat indications impacting less than 200,000 patients per year in the U.S. Of the estimated 67,000 children diagnosed with autism spectrum disorders each year in the U.S. 20,000 become minimally-verbal; QBM-001 should be able to treat 15,000 of these children. Orphan Drug designation offers clinical development benefits including tax credits and eligibility for seven years of market exclusivity in the U.S. and 10 years in Europe.
- Biomarker Validation Study Completion in Q4 2019. Currently, Q BioMed is working on finalizing its biomarker validation study with its partners, and anticipates completion of this study during the fourth quarter of 2019.
- Initiate Clinical Trial in Q1 2020. QBM-001 has completed its first phase of formulation work; the second phase of formulation is expected to be finalized in August 2019. At that time, Q BioMed will request a pre-Investigational New Drug (IND) meeting with the U.S. FDA, and similar discussions with Europe’s EMA. Based on regulatory guidance, Q BioMed anticipates filing its IND submissions, including its current clinical trial design which is planned and ready to propose, by December of this year. Clinical trials may commence as early as in the first quarter of 2020.
- Collaborators and Key Opinion Leaders in Coming Quarters. Following the discovery of biomarkers, Q BioMed has received keen interest from potential collaboration partners and thought leaders in autism. The company plans to add additional key opinion leaders in autism and PMVA to its advisory board in the coming quarters.
About Q BioMed
Q BioMed is a biotech acceleration and commercial stage company. It is focused on licensing and acquiring undervalued biomedical assets in the healthcare sector, dedicated to providing these target assets; strategic resources, developmental support, and expansion capital to ensure they meet their developmental potential, enabling them to provide products to patients in need.